Lang Suping
General Manager, GCP ClinPlus Co., Ltd.
Wu Jingfang
GCP Library Creation Team Leader
GCP ClinPlus Co., Ltd. (hereinafter referred to as “GCP” for short) is a clinical CRO in line with the ICH standard. It is dedicated to providing clinical research technology services for global pharmaceutical and medical device companies, and is the first CRO in China to provide joint development of pharmaceutical affairs between China and the United States, the European Union, Korea, J apan, Taiwan China, and other countries and regions. It is now one of the top global clinical research solution providers in China.
How to further improve organizational efficiency to ensure rapid business growth?
“There are two core aspects to ensure continuous and rapid business growth: efficient and high-quality study execution and stable and sound customer experience. Both of these must and can only be achieved through
deeper digitalization.”
–Hao Yonghong, Vice President of GCP
The average time to create a library for each study at GCP is about 8 weeks, and it takes nearly 3 months of training and learning for new people to join the study. Although in the upper tier of the industry, there is still a need for GCP to improve for its fast-growing business. In nearly 20 years of development, the GCP parametric statistics team has accumulated rich study experience. However, GCP hopes to find the answer to how experience can be formed into effective, replicable and transferable knowledge.
Taimei eCollect R Electronic Data Collection System and CRF Enterprise Library
Tamiami team assisted the GCP team to develop a customized data management solution based on eCollect, focusing on both “Enterprise CRF Standard Library Creation” and “SOP Process Optimization” to help GCP to achieve the following goals: ·Organize knowledge systematically to improve database creation efficiency
·Optimize SOP process to enhance the quality and efficiency of study execution based on system feature
Clear, smooth, and effective implementation
Create a study template library: Integrate all study information and generate nearly 50 standardized study templates (suitable for different indications and different phases), which can be reused in similar new studies at any time, thus greatly improving the efficiency of library creation.
Create a general template library: Based on Taimei enterprise template library, general templates are created for GCP studies. For the simple studies, the templates can be directly called without repeatedly building libraries. Meanwhile, the CRF Library is built, so the CRF standard sheets can be called at any time.
Customize multi-dimensional training to improve efficiency and effectiveness
· Share existing customized function codes to reduce learning costs
· Regular communication of system requirements: optimize user operation experience · Online training on system updates: ensure users master the system’s new features
· Case sharing of library creation: help the new library creators quickly get started
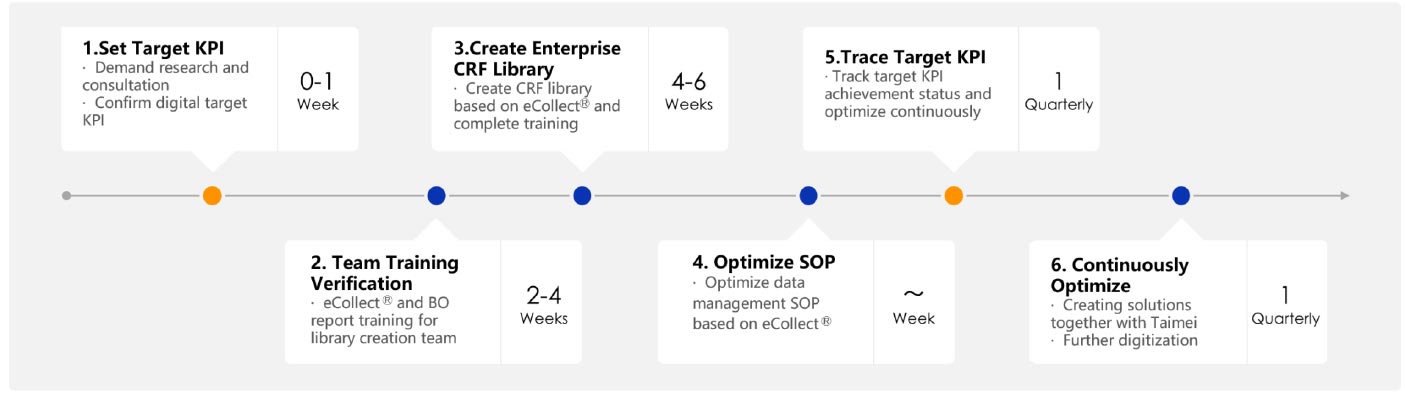
Enterprise CRF comprehensive library greatly improves the efficiency of library creation
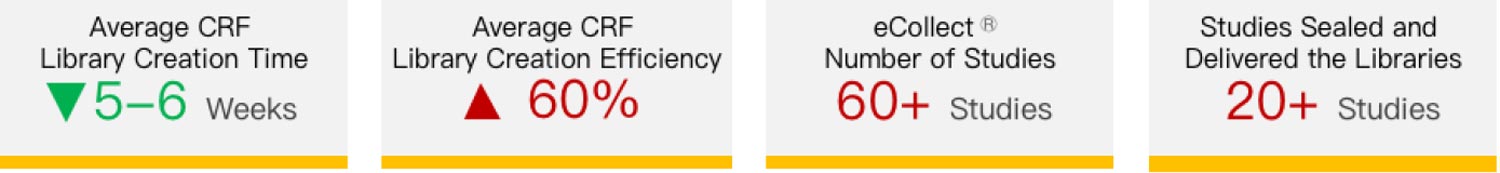
Data libraries are quickly created by referring to the templates instead of recreating them according to CDISC standards. While ensuring data quality, it significantly reduces labor, far ahead of CRO peers in the industry! Since the first template was created in April 2020, the team has created more libraries by calling templates for over 20 studies.
Optimize SOPs based on EDC features to improve organizational efficiency
Also, the use of eCollect� has allowed for significant time savings in other data management tasks such as optimizing the database migration SOP, and transforming manual migration into digital migration process They have made GCP parametric statistics team one of the few digital teams in the industry.
Customized courseware + regular sharing, team “personnel efficiency” greatly improved
Taimei provides customized and segmented online training materials, which are implemented together with GCP’s “internal training + assessment” system, making the training more effective. Monthly Workshops give team members the opportunity to share study experiences on a regular basis. Currently, 60+ projects are conducted via eCollect�, of which more than 20 projects have been sealed and delivered. The average library creation time has been shortened to 2-4 weeks, while new staff can meet the requirements to participate in studies in about 1 week. It is evident that digitalization has empowered CRO parametric statistics teams and greatly optimized the data processing process and efficiency, with the ability to flexibly respond to sponsors’ changing needs, improve clinical study quality and reduce costs. It allows CROs to form unique market competitiveness and earn reputation.
“In the era of digitalization and intelligence, as CRO, we share a common goal with platform operators to make new pharmaceutical clinical trials easier. Taimei fits our concept and goal, and with the powerful system functions and business support, we can guarantee the high quality and efficient implementation of trial studies.
In the 6 years of cooperation with Taimei, we have seen Taimei’s growth and are happy that there is such a company in China that is making digital operation better and better. ”
–Hao Yonghong, Vice President of GCP
“We have been in contact with Taimei Medical EDC since it was just released, and have been cooperating with it in depth ever since. Through Taimei EDC, we have improved the quality of data standards and the efficiency of data cleaning, as well as the efficiency of EDC library creation, which is conducive to study promotion. ”
—- Wu Jingfang,GCP Library Creation Team Leader