
Collaborative platform for clinical trials with standardized templates, real-time updates, and seamless integration with eTMF and eCollect (EDC) to track progress, control risks, & reduce costs

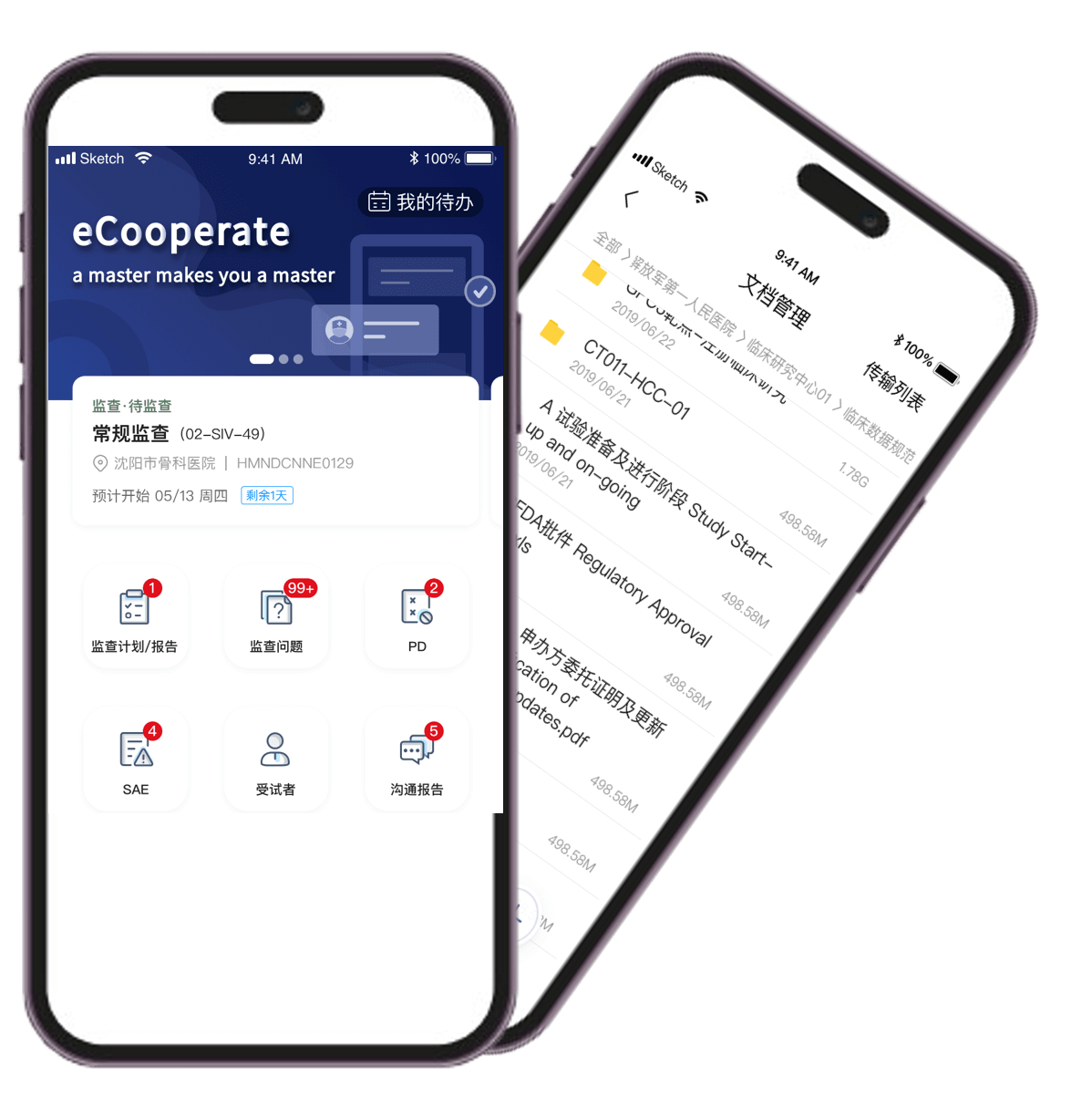
CRA task reminders, auto-generated to-do lists, and intelligent report pre-filling
PM smart approvals with automatic priority filtering
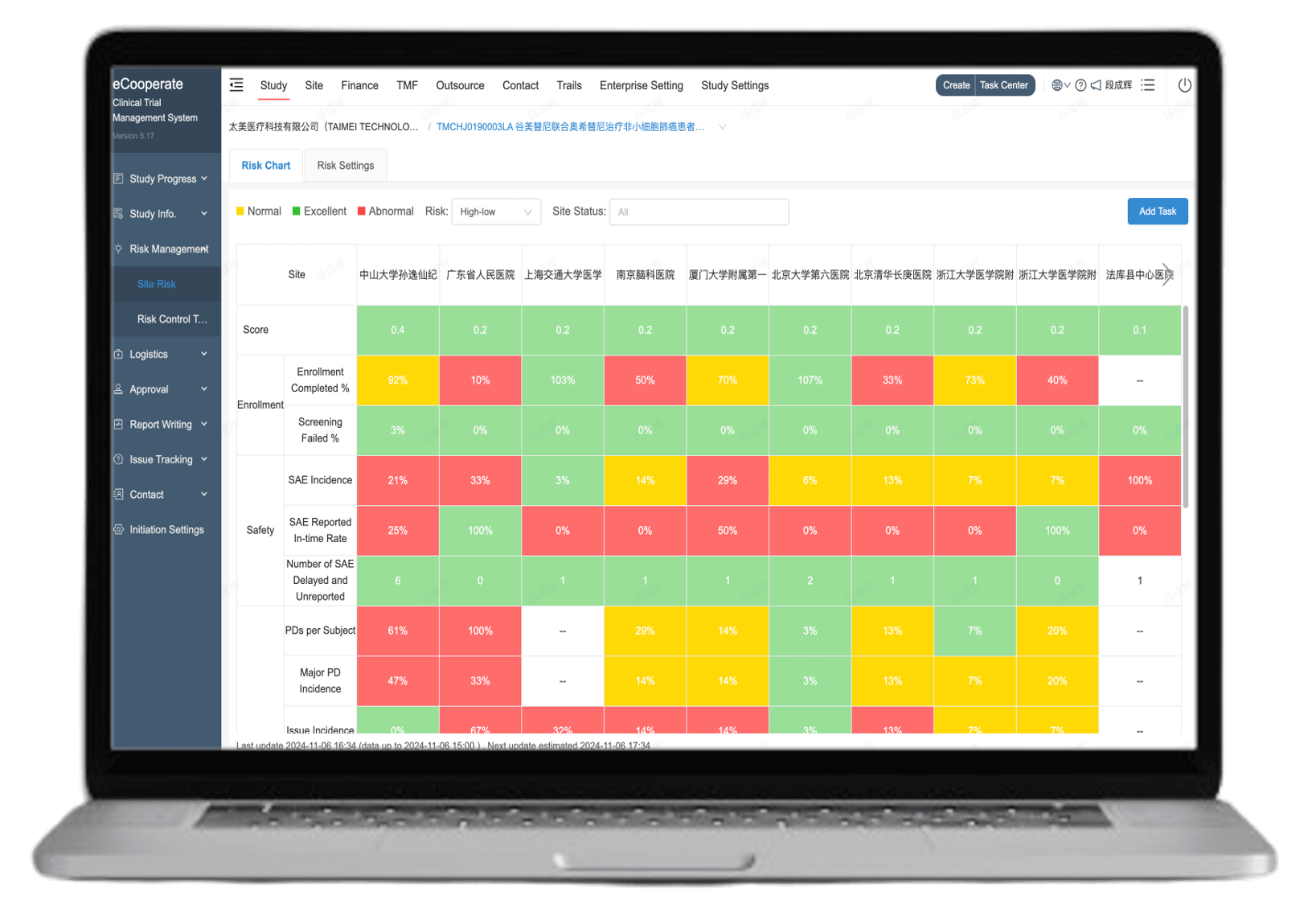
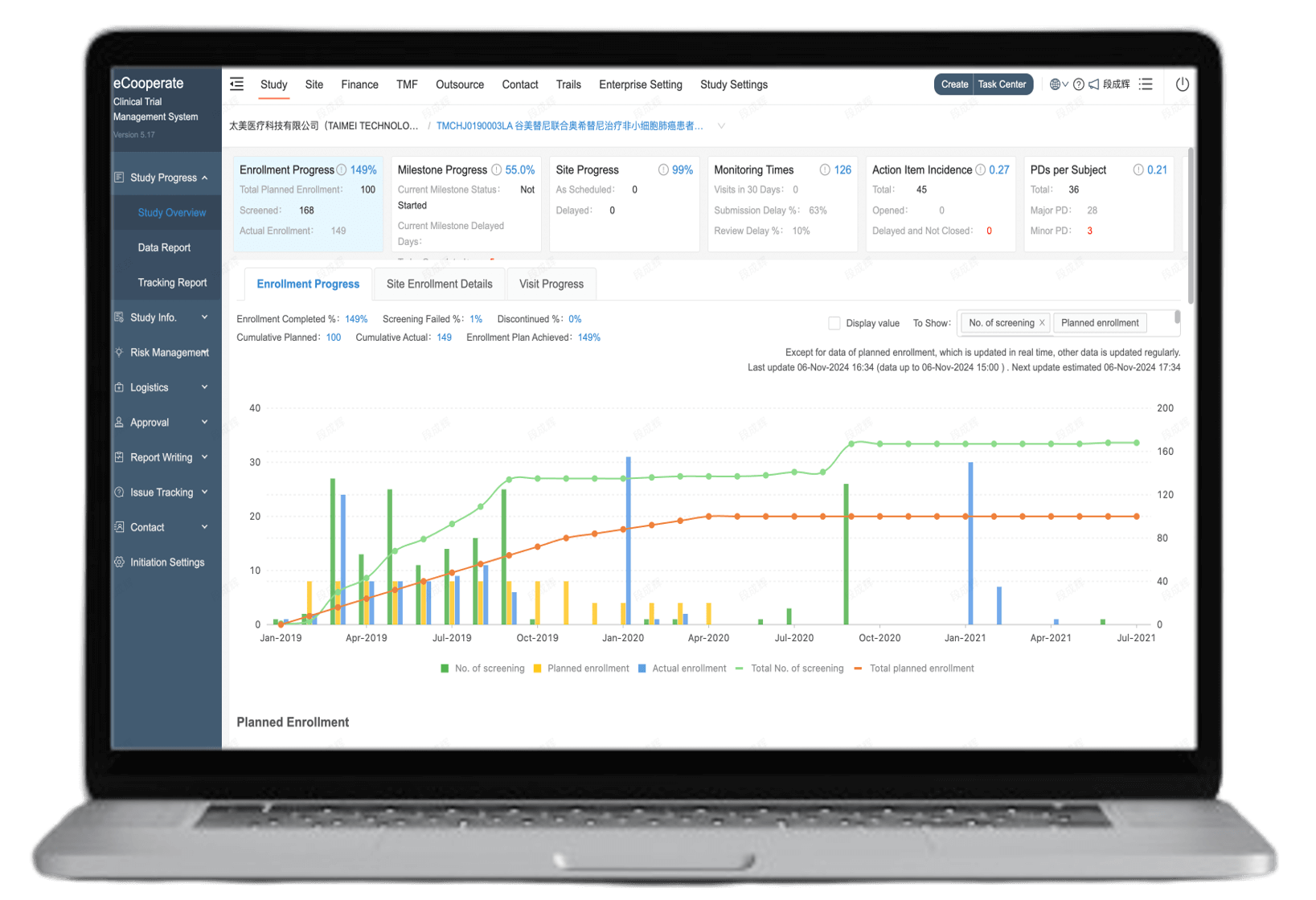
Real-time visual reports
Subject’s data entered in EDC automatically syncs to the CTMS, eliminating duplicate entries
Queries generated in EDC are automatically calculated for resolution and occurrence rates
CRA SDV rates are directly synced to CTMS for centralized management


Scan to Follow Us on LinkedIn
